Gesynta Pharma's targeted approach to the enzyme mPGES-1 provides more precise treatments for inflammation and pain.
Gesynta Pharma’s drug candidates utilize a unique mechanism of action to reduce inflammation and pain. We are currently developing two drug candidates. Vipoglanstat is in clinical phase 2. A clinical trial in patients with endometriosis was initiated in 2025. Our second drug candidate GS-073 is ready to enter clinical phase 1 for the treatment of chronic inflammatory pain. Read more about our drug development projects.
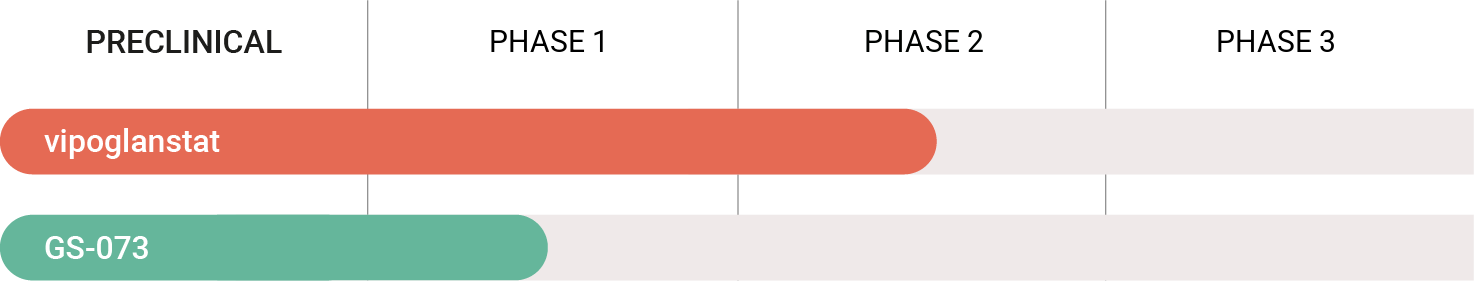
Development pipeline
Gesynta Pharma develops pharmaceutical treatments of inflammation – a key underlying driver in a spectrum of diseases. The most advanced drug candidate, vipoglanstat, is currently in development as a non-hormonal, non-opioid, disease-modifying treatment of endometriosis – a painful chronic inflammatory disease that affects about 10 percent of women of reproductive age. Vipoglanstat selectively inhibits mPGES-1, an enzyme that promotes production of the proinflammatory mediator prostaglandin E2 (PGE2). In phase 1 and 2 clinical trials, vipoglanstat has demonstrated a favorable safety profile and potent inhibition of mPGES-1. A preclinical proof-of-concept study in an advanced disease model of endometriosis showed that vipoglanstat markedly reduced both pain and the number of endometrial lesions. Based on these robust data, Gesynta Pharma has initiated a phase 2 clinical trial of vipoglanstat in patients with endometriosis.
Gesynta Pharma’s second drug candidate to reach clinical development, GS-073, targets the same enzyme as vipoglanstat. GS-073 is ready to enter clinical phase 1 for the treatment of chronic inflammatory pain.