Vipoglanstat selectively and safely inhibits mPGES-1
Gesynta Pharma develops vipoglanstat (GS-248) – a drug candidate with a unique ability to selectively and potently inhibit microsomal Prostaglandin E Synthase-1 (mPGES-1). This enzyme has been shown to play a key role not only in pain and inflammation in general, but also more specifically in endometriosis.
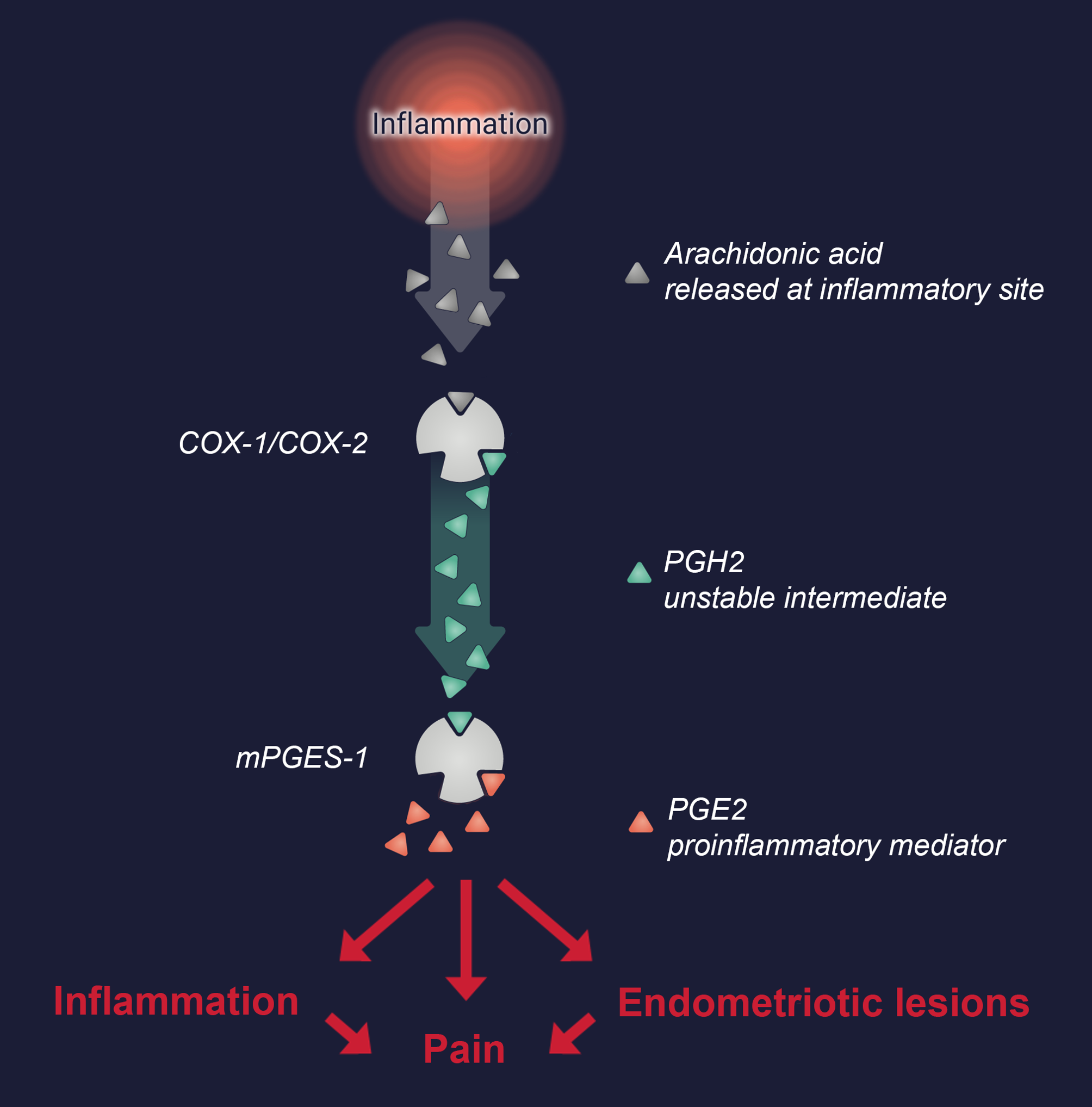
The enzyme mPGES-1 is part of the complex arachidonic acid cascade, which regulates major physiological and pathogenic processes via biosynthesis of mediators with potent biological effects, mainly at the site of production. In this cascade, mPGES-1 is upregulated to specifically catalyze the production of proinflammatory prostaglandin E2 (PGE2), which is well known to mediate pain and inflammation in a broad range of chronic inflammatory diseases, including endometriosis. It has been demonstrated that the endometriotic lesions (inflammatory tissue similar to the uterine lining growing outside of the uterus mainly in the pelvic area) contain high levels of mPGES-1, leading to increased PGE2 production. This has also been confirmed by the finding of increased PGE2 levels in the abdominal fluid from endometriosis patients. Prostaglandin E2 not only causes pain and inflammation in endometriosis but also plays a key role in endometriotic lesion development. The effect of PGE2 in the lesions is believed to be mediated through local stimulation of estrogen production and by release of angiogenetic VEGF (vascular endothelial growth factor). Both estrogen and angiogenesis are of great importance for endometriosis lesions to thrive.
In conclusion, inhibition of mPGES-1 is anticipated to decrease inflammation and reduce proliferation of endometriotic lesions by reducing proinflammatory PGE2 production, thus decreasing pain and having a disease-modifying effect.
Development of vipoglanstat
Vipoglanstat has proven preclinical proof-of-concept in an advanced disease model of endometriosis. The results show that the drug candidate markedly reduces the number of endometriotic lesions and has a positive effect on parameters measuring pain and well-being. Its effect on the endometriotic lesions points toward a disease-modifying capability of vipoglanstat.
Vipoglanstat has previously been evaluated in clinical studies in healthy subjects as well as in patients suffering from chronic inflammatory disease. The results show that vipoglanstat can be administered orally in a patient-friendly way and quickly reaches therapeutic levels in the blood. Importantly, treatment with vipoglanstat completely inhibits mPGES-1 in patients with chronic inflammation. No serious side effects were observed during four weeks of treatment and the drug candidate was safe and well-tolerated by the patients.
The compelling results from the preclinical proof-of-concept study of vipoglanstat provide strong support for its analgesic and disease-modifying capacity. Together with data from previous clinical studies regarding the drug candidate's safety profile, pharmacokinetic properties, and ability to inhibit the target enzyme mPGES-1, this constitutes a solid scientific foundation for the continued development of vipoglanstat as an effective non-hormonal treatment for endometriosis. A clinical phase II study in patients with endometriosis is now being prepared.
Intellectual property
Vipoglanstat is protected by composition of matter patents in the most important markets – the United States, Europe, China, Japan – as well as in a number of other countries. In addition to the already approved patents, a patent application was submitted in 2022 covering the use of vipoglanstat in endometriosis, to strengthen and extend the already granted IP protection.
Other indications: GS-073
In addition to vipoglanstat, Gesynta Pharma's advanced pipeline comprises a further mPGES-1 inhibitor, GS-073. This candidate drug is ready to enter clinical development for the treatment of chronic inflammatory pain, an area of significant medical need. Gesynta Pharma has developed complete preclinical safety documentation and a study protocol for the GS-073 project.
GS-073 has demonstrated a highly effective suppressive effect on both inflammation and pain in a recently conducted preclinical disease model of arthritis. The primary objective of the planned phase I study is to study the safety, tolerability, and pharmacokinetic properties of GS-073 in healthy participants as well as its effect on relevant biomarkers. This will provide important knowledge for the planning of the continued clinical program.